Product liability cases involve defective consumer products that cause injury, illness, or property damage.
If you suspect a defective product was responsible for the harm suffered by you or a loved one, our product liability attorneys are here to help. Call us at 866-510-0580 or contact us online for a free case consultation.
What if I’m Hurt by a Defective Product?
If you or a loved one is injured or becomes ill due to a product you believe may be faulty, the following steps can help preserve your ability to seek compensation through a product liability case:
- Seek emergency medical treatment as necessary
- Keep the product and any related material, such as the packaging, product instructions, and your receipt
- Make note of the circumstances in which the injury occurred
- Take photos of the injury and the site where the injury occurred
- Consult with a knowledgeable product liability lawyer
The Oklahoma City defective product attorneys at Carr & Carr have extensive experience in product liability cases, and we have helped injury victims from across the country who were harmed by dangerous consumer goods.
We offer free consultations to help you understand your rights after being injured by a defective product, and we don’t charge unless we recover compensation on your behalf.
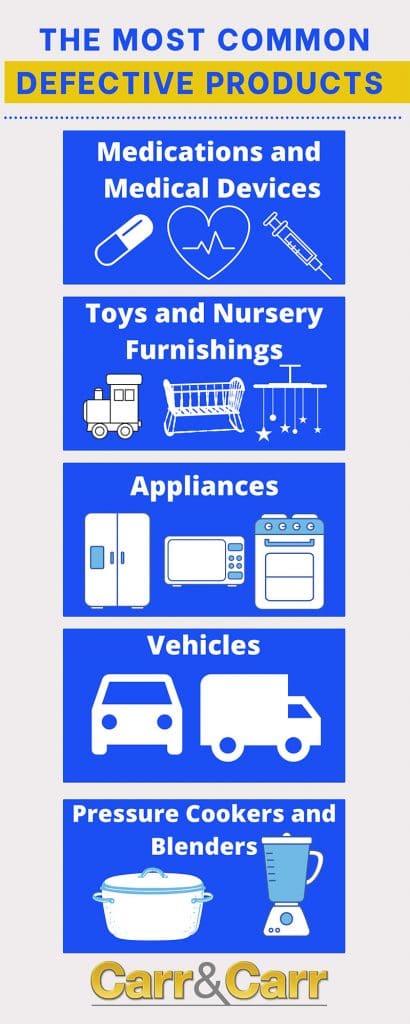
Common Types of Defective Products
Nearly any type of consumer good can pose an illness or injury threat, but there are a handful of product categories that are commonly the focus of product liability lawsuits:
- Medical devices
- Medications
- Motor vehicles and motor vehicle parts
- Children’s toys
- Appliances
- Hope Chest
- Furniture
- Food
Consumer goods that are currently the subject of product liability lawsuits include:
- Pressure Cookers, Instant Pots
- Cribs & Bassinets
- Boppy Pillows
- Button batteries
- Fisher-Price Rock ‘n Play sleepers
- Food Processors and Blenders
- Surgical hernia mesh
- Torch fuel
- Vornado electric space heaters
- NutriBullet
- Zantac
- Talcum Powder
- Window Blinds
- Paraquat
Are you a new parent or thinking about family planning?
Click here to learn how to baby proof your nursery!
Factors in Product Liability Claims

Although the range of products that can cause harm is broad, most product liability cases relate to one of three types of flaws: design defects, manufacturing defects or warning defects.
Understanding a little about the three basic types of product defects can help you determine whether you may have a valid product liability claim:
- Design defects: Flaws in a product’s design make it unreasonably dangerous
- Manufacturing defects: Faults in the manufacturing process contaminate the product or otherwise make it unreasonably dangerous
- Warning defects: The manufacturer or seller of the product fails to provide adequate warnings about the product’s risks or instructions for the product’s proper use
It’s important to note a successful product liability case must establish the product was defective and the product defect caused the resulting injury or illness.
Elements of Product Liability Cases
The plaintiff of a product liability case is the individual, or individuals, who suffered harm caused by a defective product.
The defendant is typically the product’s manufacturer, but depending on the nature of the defect and the circumstances of the resulting harm, the defendant or defendants in a product liability case may include the product’s designer, supplier, distributor, marketer, seller or repairer.
To recover damages in a product liability case, you must prove four key elements:
- You were injured or suffered losses
- You were using the product in a foreseeable way
- The product in question is defective based on at least one of the factors detailed above
- The defect caused your injury or illness
As with other types of personal injury cases, time is of the essence in product liability claims. Injury victims must file lawsuits within the applicable statute of limitations, which is a deadline to pursue a legal claim. In Oklahoma, plaintiffs have two years from the date they knew or should have known about their injury or illness to file a product liability lawsuit.
Damages in Product Liability Claims

Compensation for injury victims in product liability cases may include money for:
- Medical expenses
- Lost wages or loss of earning potential
- Pain and suffering
- Disability
If a loved one died due to a defective product, our attorneys may be able to help surviving family members seek compensation through a wrongful death case.
Oklahoma Product Liability Attorneys with a National Reach
The accomplished team of injury attorneys at Carr & Carr is dedicated to helping victims of defective products recover the financial security they need to restore their lives.
We proudly serve clients from Oklahoma and northwest Arkansas via our offices in Oklahoma City, Tulsa, and Springdale, AR. In cases related to defective products, we can represent clients across the country.
If you or a loved one suffered harm due to a potentially defective consumer good, please call us at 866-510-0580 for your free case consultation or contact us online to tell us your story now.